Gene expression profiling of macrophages: Implications for an immunosuppressive effect of dissolucytotic gold ionsThe authors point to the use of gold as a “noble” metal in medicine over a long time. Gold salts have been used in treating rheumatoid arthritis (RA), the response to it, however, is unpredictable and side effects often occur, so that they have been replaced by biologicals such as TNF-
“Macrophages express [cell] adhesion molecules, chemokine receptors and other surface antigens and secrete a number of chemokines, cytokines, growth factors, proteases and other mediators. Macrophages and their products are key players in the pathogenesis of RA and other inflammatory diseases and may be promising therapeutic targets.” By means of autometallography (AMG) the “movement of gold ions from the dissolution membrane to the intercellular environment” has been observed, from where “they enter macrophages, mast cells and other cells in the local environment”. “It has also been noted that ionisation occurs more rapidly in inflamed tissue than in normal tissue, and that the greater the surface area the greater the ionisation of gold.” The aim of the authors was to study whether the growing of human macrophages of the cell line TCP-1 on 24-carat gold foils could lead to a release of gold ions and cellular incorporation, and whether “this process was associated with changes in gene expression and subsequent protein secretion and cell viability”. For this purpose, the authors cultivated TCP-1 cells and grew them on small gold foils. After 1, 2, 3 and 4 days they were harvested and the intracellular gold uptake analysed by AMG. The cells were suspended on microscopic slides, the gold was reduced by ionisation using UVA light, silver-enhanced by AMG, where silver is deposited on gold nanoparticles. The impact of phagocytised gold ions on cell viability was analysed by trypan-blue straining and analysed by TUNEL assay. The global gene expression profile of the THP-1 cells after gold absorption was studied. RNA was gathered and controlled, subjected to Cy3-labelling, hybridised and scanned. Expression values were calculated and statistically analysed. “Genes significantly expressed (ANOVA, p < 0.05) and with a fold change
Results: AMG showed increased intracellular uptake of gold ions (dissolucytosis) in cultivated THP-1 cells after 4 days, but no significant uptake after 1, 2 or 3 days. No significant effect on the viability of the THP-1 cells was found: The cell viability with and without gold was > 95% according to the trypan method, and 14.1% and 13.2%, respectively, of the cells were TUNEL-test positive. This suggests that the “anti-inflammatory effects might not be mediated via macrophage cell death”. The “data revealed a unique gene expression signature of dissolucytotic THP-1 cells that had taken up gold ions“ (from 20.000 genes investigated 1028 (fold change (FC)
The investigation results of this study “offer new insights into the mode of action of gold ions and suggest [steps] for the investigation of effects on other key cells”, e.g. T- and B-lymphocytes, “and a possible future role of metallic gold as implants in rheumatoid arthritis or other inflammatory conditions”.
|


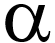 inhibitors. Metallic gold implants have also been used in veterinary medicine for a long time. Released gold ions have been shown to be anti-inflammatory. The mechanism of action, however, is not yet fully understood. It has been demonstrated that gold ions inhibit the lysosomal enzymes of phagocytotic cells, decrease the number of macrophages in the synovial membrane and lower the production of pro-inflammatory cytokines in cell cultures. “Danscher was the first to show that charged gold atoms are released from pure metallic gold implants.” His team “demonstrated that macrophages home in on the bio-membrane that covers metallic gold implants. Gold ions are released from the metallic gold surface into the ‘dissolution membrane’ under the sway of the adhering macrophages.”
inhibitors. Metallic gold implants have also been used in veterinary medicine for a long time. Released gold ions have been shown to be anti-inflammatory. The mechanism of action, however, is not yet fully understood. It has been demonstrated that gold ions inhibit the lysosomal enzymes of phagocytotic cells, decrease the number of macrophages in the synovial membrane and lower the production of pro-inflammatory cytokines in cell cultures. “Danscher was the first to show that charged gold atoms are released from pure metallic gold implants.” His team “demonstrated that macrophages home in on the bio-membrane that covers metallic gold implants. Gold ions are released from the metallic gold surface into the ‘dissolution membrane’ under the sway of the adhering macrophages.” 2 were considered differentially regulated.” The gene expression data were confirmed by measuring secreted proteins. “Early growth response 1 (EGR1), fatty acid desaturase 1 (FADS1) and lymphotoxin B (LTB) were measured”. The results of the data analysis were expressed as mean ± standard deviation, differences between the groups [without/with gold] analysed using the student t-test, and values p < 0.05 were considered significant.
2 were considered differentially regulated.” The gene expression data were confirmed by measuring secreted proteins. “Early growth response 1 (EGR1), fatty acid desaturase 1 (FADS1) and lymphotoxin B (LTB) were measured”. The results of the data analysis were expressed as mean ± standard deviation, differences between the groups [without/with gold] analysed using the student t-test, and values p < 0.05 were considered significant.